Subcutaneous immunotherapy (SCIT) has been used for more than a century for patients with allergic rhinitis, allergic conjunctivitis and/or asthma.(1) There is a “progressive mandate” to further standardize SCIT clinical practice that optimizes quality and patient safety.(2) “It is incumbent upon allergists preparing extract treatment sets for patients to be familiar with and adopt training, procedures and safety measures that lead to standardized high-quality products.”(2)
SCIT is considered safe, although adverse reactions to allergen immunotherapy do occur, states the American Academy of Allergy, Asthma, and Immunology.(4) “Although very rare, deaths associated with immunotherapy may be due to clerical and medical errors by healthcare personnel. Examples include administering a wrong dose or wrong extract to the wrong patient.”(4)
Clear and consistent labeling helps prevent errors and is a critical component of SCIT safety. USP 797 Guidance for Pharmaceutical Compounding Sterile Preparations states that vial labels must display:
- Patient name
- Type and fractional dilution of each vial, with a corresponding vial number
- BUD
- Storage conditions(3)
The American Academy of Allergy, Asthma, and Immunology also underscores the importance of clear labeling for treatment vials, stating, “Standardizing allergen immunotherapy label contents and vial coding will improve communication between care providers and patients, and likely prevent errors in extract administration.”(4) In addition to information listed above, the organization’s guidelines state that labels must include two patient identifiers, e.g., name and date of birth.(4)
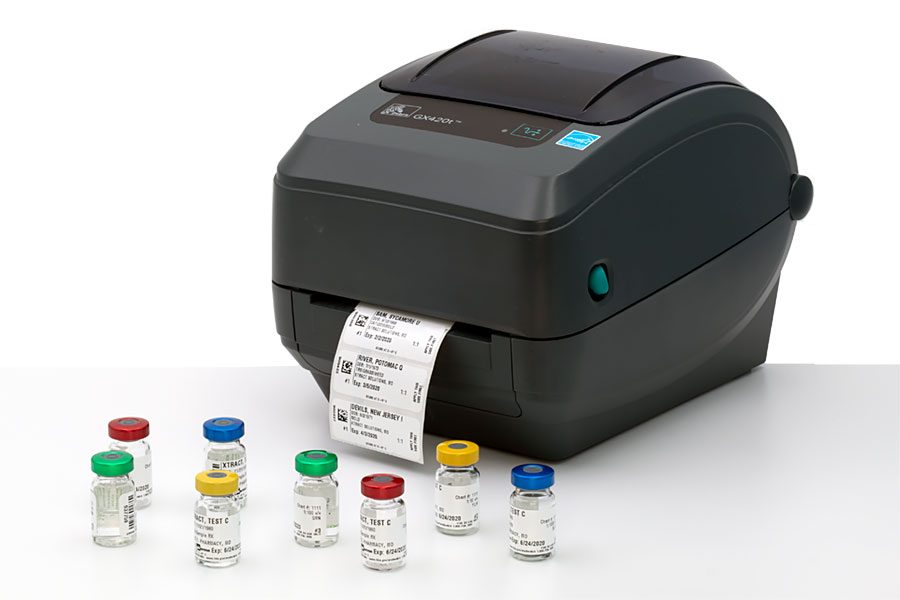
Xtract Immunotherapy Software creates clearly printed labels on water and alcohol resistant material. Barcoded labels contain patient name, date of birth, serum, dilution, BUD, and storage requirements. Xtract’s labeling software was specifically designed to print labels for compounded allergy treatment vials. Printed labels save time and improve safety, helping ensure the right injection and the right dilution for the right patient, every time.
(1) American Academy of Allergy, Asthma, and Immunology, Safety and Standardized Allergen Subcutaneous Immunotherapy in Pediatric Patients, https://www.aaaai.org/
(2) Nelson, M. M.D., Petersen, M. M.D., et. al., Current Allergy and Asthma Reports, Allergen Immunotherapy Extract Set Preparation: Making a Safe and Higher Quality Product for Patients, https://link.springer.com/
(3) USP 797, Guidance for Pharmaceutical Compounding – Sterile Preparations, https://www.usp.org/
(4) American Academy of Allergy, Asthma, and Immunology, Practice Management Resource Guide, Chapter 9, Allergy Immunotherapy Extract Preparation Manual, https://www.aaaai.org/